From Medscape Infectious Diseases > Expert Reviews and Commentary
Treating Influenza and Herpes Viral Infections
A Primer on Antiviral Agents
The world of antiviral drugs is rapidly changing with over 20 antiviral agents and 25 antiretroviral agents currently approved by the US Food and Drug Administration. Key to appropriately utilizing these agents is an understanding of the generic viral life cycle and the places points in that cycle where antivirals can act.To simplify this, there are 2 major factors that distinguish viruses:
- DNA vs RNA viruses; and
- Envelope vs nonenvelope viruses.
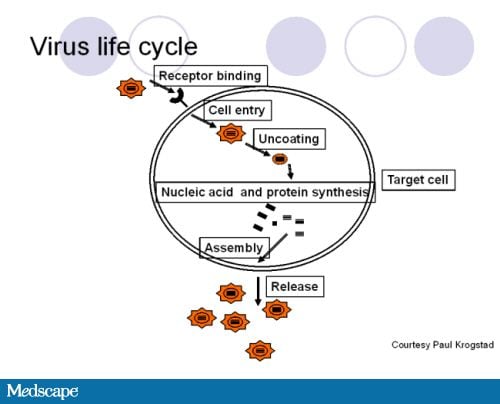
Figure 1. Viral life cycle.During the viral life cycle, a virus traffics in, binds to its receptor, enters, uncoats, makes its own nucleic acids and proteins, and then reassembles before releasing into the host system. That pretty much applies to all viruses.
Figure courtesy of Paul Krogstad, University of California, Los Angeles.
Antiviral agents target several places within this process.
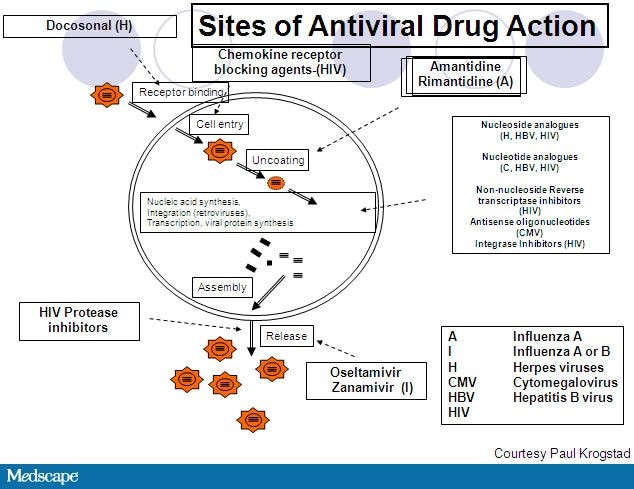
Figure 2. Sites of antiviral drug action.It is important to emphasize that the viral life cycle results in the ultimate release of new virus. Therefore, a cell that is infected is generating a lot of virions. Virology should be thought of in terms of total numbers of virus. An antiviral agent that is 99.9% effective at reducing virus will leave 0.01% of the infected cells, and these infected cells continue to make new virions. If the original infection caused 1 billion new virions to be produced, 0.01% will continue to produce 1 million new virions, which is enough to generate symptoms in a patient. The best treatment for a viral infection, therefore, is to prevent a cell from being infected. Once a cell is infected and generating new virions, an antiviral must be extremely potent in order to arrest the infection.
Figure courtesy of Paul Krogstad, University of California, Los Angeles
Antivirals for Treatment of Influenza in Children
There are 4 antiviral agents currently available for use in both influenza A and B infections. All target a viral neuraminidase that is critical for release of the virus. The agents approved for this indication are:Amantadine. This agent inhibits viral uncoating, a critical step in viral replication. Administered orally, it is almost 100% bioavailable. It is only effective against influenza A strains. Similar to other agents, it reduces both severity and duration of symptoms if given early in the course of the infection. It is associated with adverse central nervous system (CNS) effects, most notably jitteriness. More severe CNS symptoms may include hallucinations or insomnia.
Rimantadine. Rimantadine, developed later than amantadine, is associated with somewhat less CNS adverse effects.
Unfortunately, over the last 15 years, influenza viruses, including novel H1N1 and H3N2, are both widely resistant to amantadine or rimantadine. Currently, approximately 92% of influenza strains are resistant. The Centers for Disease Control and Prevention (CDC) do not recommend use of either agent in treatment of influenza.[1]
Oseltamivir. This agent is well absorbed and thus readily bioavailable when given orally. The target for this agent is viral neuraminidase, which is critical for viral release. While active against both influenza A and B, it is approximately 10- to 20-fold more active against A than B. Efficacy studies demonstrate that antivirals initiated in the first 2 days of an influenza infection, including oseltamivir, reduce the severity of symptoms as well as duration by approximately 1 day, a reflection of the earlier discussion on the high volume of new virions made during a viral infection.
Zanamivir. Zanamivir is an inhaled powder administered via a Diskhaler. Due to risk for bronchospasm, this agent may not be used in patients with a history of asthma. There is an investigational intravenous formulation in development. Like oseltamivir, this agent targets viral neuraminidase and should be administered within the first 2 days of symptom onset. Recommendations for use of oseltamivir and zanamivir are available from the CDC Website.
Treatment of Herpes in Children
Herpes viruses represent a large family of DNA viruses known for their ability to cause lifelong infection. In aggregate, these viruses are associated with a number of distinct conditions causing significant human morbidity. The most clinically important of these viruses include:- Cytomegalovirus (CMV) -- typically, healthy persons infected with CMV are asymptomatic but infection can be life-threatening in the immunocompromised patient.
- Epstein-Barr virus (EBV) -- most widely recognized as the cause of infectious mononucleosis, EBV is also associated with some forms of cancer, particularly Burkitt lymphoma and nasopharyngeal carcinoma.
- Herpes simplex virus (HSV) 1-- may cause oral or genital lesions but is primarily associated with orofacial infection.
- HSV2 -- also may be the cause of either oral or genital lesions, though primarily associated with genital infection.
- Varicella zoster virus (VZV) -- causes chickenpox as a primary infection and may later reactivate as the cause of shingles.
Acyclovir and valacyclovir. These agents are guanosine analogs that dead end DNA replication by competitively inhibiting the viral polymerase. The oral bioavailability of acyclovir is quite low, particularly for pediatric suspensions. Valacyclovir is better absorbed, though bioavailability remains modest at between 55%-70%. Side effects are typically minimal, though renal toxicity occurs in approximately 5% of patients, with a slightly higher incidence in children particularly those with underlying renal insufficiency or who may be taking other nephrotoxic medications. Intravenous preparations may cause local irritation and phlebitis. Neurotoxicity, presenting with symptoms ranging from agitation to frank psychosis, can occur in 1%-4% of patients, primarily in adults. While acyclovir is present in breast milk there is no known teratogenecity.
Ganciclovir and valganciclovir. The mechanism of action of these agents is similar to that seen with acyclovir though the drugs are less efficient at chain termination. Ganciclovir has very poor bioavailability though valganciclovir is approximately 60% bioavailable. Valganciclovir specifically inhibits bone marrow hematapoietic precursor cells, which is the reason that neutropenia, which occurs in 24%-43% of patients, and thrombocytopenia, seen in 5%-15% of recipients, are prominent adverse effects associated with this agent. CNS side effects, ranging from headache to behavior changes, may also be seen with either of these agents. Both agents are teratogenic and classified as Pregnancy Category C and thus are contraindicated during breast feeding. These agents have particular usefulness in the treatment of CMV infection in those patients requiring treatment.
Famciclovir and penciclovir. Penciclovir is a related compound to acyclovir with activity against HSV1 and 2 as well as VZV. It is not orally bioavailable and is used topically. Famciclovir is the oral prodrug of penciclovir and can be used orally for treatment of cutaneous/mucosal manifestations of HSV or VZV.
Foscarnet. This agent is an inorganic pyrophosphate analog that is a noncompetitive inhibitor of the viral polymerase and interferes directly with the enzyme. It has broad activity against herpes viruses and is used primarily in the situation of resistant HSV and CMV infections. It does not have any oral bioavailability and must be given intravenously. The major side effect is renal toxicity; one third of patients will double their serum creatinine by week 2 of treatment. Risk factors for renal toxicity include use of higher doses, rapid infusion, and the presence of other nephrotoxic medications. Because the drug is primarily excreted renally, it requires dose adjustment for patients with renal compromise. Because foscarnet chelates many other agents, it is not compatible with many other infusions. CNS side effects are also common, occurring in approximately 25% of patients and ranging from headache to frank dystonia. It is teratogenic, and it is excreted in breast milk.
Cidofovir. Cidofovir is an acyclic phosphonate nucleotide analog of the deoxycytidine that is a broad inhibitor of many DNA viruses. It has activity in vitro against HSV, CMV, EBV, human herpes virus 6 and 8, and a range of other viruses including some papillomviruses, polyomaviruses, adenoviruses, and poxviruses. It, too, has low oral bioavailability, only about 5%, so it must be administered intravenously.
It is almost entirely excreted unchanged in the urine. Probenecid coadministration prolongs the half life. Nephrotoxicity is a major limiting factor in the use of this drug because it binds to the anti-transporter within the kidney which leads to drug accumulation in the renal cortex. It is mutagenic and teratogenic. It is classified Pregnancy Category C, and it is present in breast milk.
1 comment:
I am from USA. I was suffering from HEPATITIS B for over 3 years, i was hopeless until one of my friend directed me to a herbal DR. Dr Chike on Youtube, she said the Dr has herbal medicine that treat HEPATITIS B also said the Dr has helped people with. HERPES, CANCER, DIABETES, HPV, HERPES, HSV 1 .2, Fever, Fibromyalgia, Fatigue and chronic pains. At first I never believed her but after a lot of talk. I decided to contact him, just few days ago i contacted him and he told me what to do which i did and he sent to me a herbal medicine via {DHL} with prescriptions on how i will take it for a period of days. After i finished taking the medicine for 2 weeks he told me to go for a test which i also did and when the result came out i was surprised to see that i am negative. I am proud to tell you that I am the happiest person on earth. Big thanks to Dr Chike herbs .. I pray you find a solution in him. For more information on how to get treated Contact Dr on WhatsApp . +233502715551, or Facebook page, @ Dr Chike Herbal Remedy.
Post a Comment