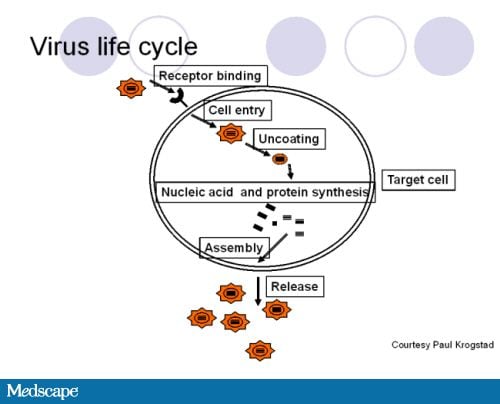
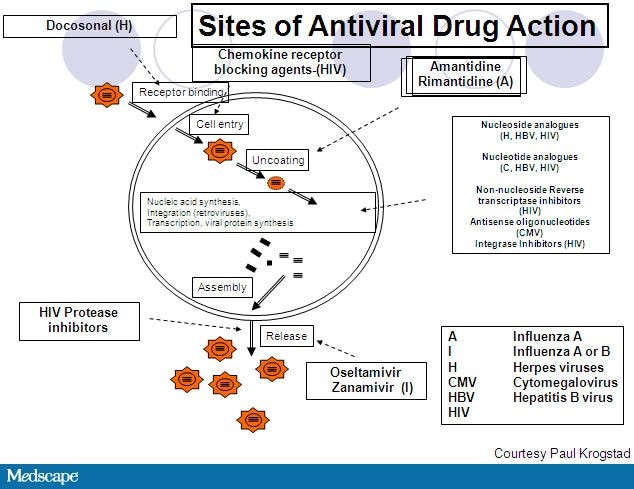
July 27, 2011 — New guidelines from the American Academy of Pediatrics (AAP) provide clinicians with the latest recommendations on the health supervision of children with Down syndrome.
The guidelines, published online July 25 in Pediatrics, provide standards of care beginning with the prenatal diagnosis of Down syndrome and extending through age 21 years or older.
"The guidelines are directed to the primary care provider to help meet the needs of the child with Down syndrome in the medical home," lead author Marilyn J. Bull, MD, FAAP, the Morris Green Professor of Pediatrics at Riley Hospital for Children in Indianapolis, Indiana, told Medscape Medical News.
In the guidelines, Dr. Bull and the Committee on Genetics state that children with Down syndrome have an array of unique medical conditions, multiple malformations, and widely varying levels of cognitive impairment, and clinicians are advised to be aware of the various issues that arise because of the presence of extra genetic material from chromosome 21.
As many as 75% of these children have hearing loss, for instance, and as many as 50% have congenital heart disease, Dr. Bull explained.
"It's important for physicians to recognize and manage potentially disabling conditions such as thyroid and cardiology issues as early as possible."
Among key updates to the guidelines are recommendations on monitoring children for cervical spine disabilities. In the past, radiographs of the cervical spine were recommended for children with Down syndrome by approximately age 3 years to detect the risk for instability that can cause spinal compression or spinal cord damage, but Dr. Bull said there is question about the efficacy of the radiographic approach.
"The problem is the radiographs themselves are not a good predictive tool and they don't predict well which child is going to have that increased risk of cervical spine instability," she said.
"So we are placing a different emphasis toward alerting physicians and families of the symptoms that could relate back to the cervical spine and to the prompt intervention that's important should they occur."
The guidelines also now include recommendations that clinicians be on alert for celiac disease, or gluten sensitivity, in children with Down syndrome.
"Celiac disease is somewhat increased in some children with Down syndrome, and since there are potential burdens in evaluating asymptomatic children, we don't recommend every child needs to be evaluated," Dr. Bull said. "But we're recommending that clinicians have a heightened awareness of the symptoms and conduct the proper tests if those symptoms occur."
Sleep apnea is another risk in children with Down syndrome because of anatomic predisposition, and testing is recommended by at least age 4 years or as soon as a child has symptoms, Dr. Bull noted.
The guidelines include an algorithm to help address the various age-specific health issues that can arise throughout growth and development with Down syndrome.
"As children mature, nutrition, behavior and sexuality all become important issues in managing these patients, and health guidance on the issues for families is addressed as well," Dr. Bull said.
Family interaction in health supervision at all ages is heavily emphasized throughout the guidelines, which stress the need to work with parents to help them adjust to the unique challenges of having a child with Down syndrome.
She explained that "Parents are often overwhelmed when they learn their baby has a condition such as Down syndrome, and these guidelines strongly emphasize how important a balanced perspective with accurate information is for families, and how [to] approach families with the appropriate sensitivity, because it certainly is an adjustment."
Although much health supervision of patients with Down syndrome is handled at the primary care level, the guidelines are ultimately designed to inform and benefit the broader array of clinicians that can also be involved in their care.
"Almost every specialty is involved in caring for children with Down syndrome, so it is important for residents and medical students to become familiar with [Down syndrome] because it is highly likely they will encounter a Down syndrome patient in the course of their careers," Dr. Bull said.
Dr. Bull has disclosed no relevant financial relationships.
Pediatrics. 2011;128:393-406.
The guidelines, published online July 25 in Pediatrics, provide standards of care beginning with the prenatal diagnosis of Down syndrome and extending through age 21 years or older.
"The guidelines are directed to the primary care provider to help meet the needs of the child with Down syndrome in the medical home," lead author Marilyn J. Bull, MD, FAAP, the Morris Green Professor of Pediatrics at Riley Hospital for Children in Indianapolis, Indiana, told Medscape Medical News.
In the guidelines, Dr. Bull and the Committee on Genetics state that children with Down syndrome have an array of unique medical conditions, multiple malformations, and widely varying levels of cognitive impairment, and clinicians are advised to be aware of the various issues that arise because of the presence of extra genetic material from chromosome 21.
As many as 75% of these children have hearing loss, for instance, and as many as 50% have congenital heart disease, Dr. Bull explained.
"It's important for physicians to recognize and manage potentially disabling conditions such as thyroid and cardiology issues as early as possible."
Among key updates to the guidelines are recommendations on monitoring children for cervical spine disabilities. In the past, radiographs of the cervical spine were recommended for children with Down syndrome by approximately age 3 years to detect the risk for instability that can cause spinal compression or spinal cord damage, but Dr. Bull said there is question about the efficacy of the radiographic approach.
"The problem is the radiographs themselves are not a good predictive tool and they don't predict well which child is going to have that increased risk of cervical spine instability," she said.
"So we are placing a different emphasis toward alerting physicians and families of the symptoms that could relate back to the cervical spine and to the prompt intervention that's important should they occur."
The guidelines also now include recommendations that clinicians be on alert for celiac disease, or gluten sensitivity, in children with Down syndrome.
"Celiac disease is somewhat increased in some children with Down syndrome, and since there are potential burdens in evaluating asymptomatic children, we don't recommend every child needs to be evaluated," Dr. Bull said. "But we're recommending that clinicians have a heightened awareness of the symptoms and conduct the proper tests if those symptoms occur."
Sleep apnea is another risk in children with Down syndrome because of anatomic predisposition, and testing is recommended by at least age 4 years or as soon as a child has symptoms, Dr. Bull noted.
The guidelines include an algorithm to help address the various age-specific health issues that can arise throughout growth and development with Down syndrome.
"As children mature, nutrition, behavior and sexuality all become important issues in managing these patients, and health guidance on the issues for families is addressed as well," Dr. Bull said.
Family interaction in health supervision at all ages is heavily emphasized throughout the guidelines, which stress the need to work with parents to help them adjust to the unique challenges of having a child with Down syndrome.
She explained that "Parents are often overwhelmed when they learn their baby has a condition such as Down syndrome, and these guidelines strongly emphasize how important a balanced perspective with accurate information is for families, and how [to] approach families with the appropriate sensitivity, because it certainly is an adjustment."
Although much health supervision of patients with Down syndrome is handled at the primary care level, the guidelines are ultimately designed to inform and benefit the broader array of clinicians that can also be involved in their care.
"Almost every specialty is involved in caring for children with Down syndrome, so it is important for residents and medical students to become familiar with [Down syndrome] because it is highly likely they will encounter a Down syndrome patient in the course of their careers," Dr. Bull said.
Dr. Bull has disclosed no relevant financial relationships.
Pediatrics. 2011;128:393-406.